Alkyne beads
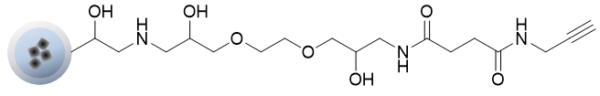
Alkyne beads can be easily immobilized on beads by a click chemistry reaction of compounds with azide groups.
In the case of a compound having multiple immobilizable sites such as an amino group, the compound can be immobilized on the beads in a site-specific manner by introducing an azide to the site to be immobilized.

Magnetic beads
The lineup of this product is only regular FG beads.
| Beads | FG beads |
|---|---|
| Code | TAS8848N1161 |
| Price | Please contact us |
| Storage conditions | 2-8 ℃ (no freezing) |
| Storage buffer | Ultrapure water |
| Magnetization | Superparamagnetism (≧10 emu/g) |
| Size of beads | 180±30 nm |
| Concentration | 20 mg/ml |
| Functional groups | Ethynyl group |
| Amounts of the functional groups | 70-140 nmol/mg of beads |
- Protocol
- SDS
- Papers /
Technical Information - Related Products
- FAQ
- Screening by using ligand immobilized beads
- Competitive inhibition
- Drug elution
- Immobilization of ligands (azide structure compounds) on alkyne beads using click chemistry reaction
- Immobilization of ligands (azide structure compounds) on alkyne beads using click chemistry reaction (Small scale method)
- Preparation of cell extract (Large scale method)
- Preparation of cell extract (Small scale method)
Technical Information
papers
-
Takanori Shinotsuka et al.(2023)
Stimulated Raman scattering microscopy reveals a unique and steady nature of brain water dynamics
Cell Reports Methods 3, 100519 July 24, 2023 -
Direct Visualization of General Anesthetic Propofol on Neurons by Stimulated Raman Scattering Microscopy
iScience 25, 103936, March 18, 2022 -
Adenine nucleotide translocase 2, a putative target protein for 2-carba cyclic phosphatidic acid in microglial cells
Cellular Signalling 82 (2021) 109951 -
Vizantin Inhibits Endotoxin-Mediated Immune Responses via the TLR 4/MD-2 Complex
J. Immunol., 193, 4507 (2014). -
Protein fishing using magnetic nano-beads containing calmodulin site-specifically immobilized via an azido-group
J. Biochem., DOI:10.1093 (2013).
- Please tell me how to separate FG beads (magnetic separation and centrifugation).
- Please tell me how to disperse FG beads (ultrasonic method and manual method).
- I mistakenly frozen some beads that were supposed to be stored in the refrigerator. Is it available?
- What amount of the beads is required?
- What are the important points when designing a ligand?
- How are beads stored after the ligands are bound to them?
- Are there any methods other than HPLC for verifying whether or not ligand binding has been successful?
- How strong is the affinity for the proteins that are affinity purified?
- What is the purification efficiency?
- How is the cell extract prepared?
- Is there any problem with using frozen stock homogenate?
- How much protein supply is necessary?
- Can affinity purification be used with membrane proteins such as GPCRs and ion channels?
- There are many background bands. how can i reduce it?
- What should be done when a large number of bound protein bands are detected?
- Is it necessary to use the recommended buffer as the binding buffer?
- Why is it that both salt elution and boil elution are performed for elution?
- Does it happen that the band of bound protein becomes thin when the concentration of ligand is increased?
- Why can’t I see any bands of bound proteins?
- How long is the stable period of the ligand-immobilized beads?
- Is the optimal binding reaction time of 4 hours?
- I want to analyze bound proteins with MS, but what should I do if the target protein band is thin?
- How much protein can be analyzed by MS?

