OH beads
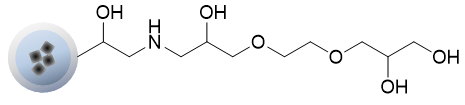
OH beads can immobilize highly reactive COOH groups directly on the beads.
Although the reactivity is low, non-specific adsorption is extremely low, and unreacted OH groups do not need to be masked.
It is used when the masking treatment of NH2 beads affects the compounds.

Magnetic beads
The lineup of this product is only regular FG beads.
| Beads | FG beads |
|---|---|
| Code | TAS8848N1120 |
| Price | Please contact us |
| Storage conditions | 2-8 ℃ (no freezing) |
| Storage buffer | Ultrapure water |
| Magnetization | Superparamagnetism (≧10 emu/g) |
| Size of beads | 180±30 nm |
| Concentration | 20 mg/ml |
| Functional groups | Hydroxy group |
- Protocol
- SDS
- Papers /
Technical Information - Related Products
- FAQ
- Screening by using ligand immobilized beads
- Immobilization of ligands (carboxylic compounds) on OH beads
- Immobilization of ligands (carboxylic compounds) on OH beads (Small scale method)
- Competitive inhibition
- Drug elution
- Preparation of cell extract (Large scale method)
- Preparation of cell extract (Small scale method)
Technical Information
Papers
- Please tell me how to separate FG beads (magnetic separation and centrifugation).
- Please tell me how to disperse FG beads (ultrasonic method and manual method).
- I mistakenly frozen some beads that were supposed to be stored in the refrigerator. Is it available?
- What amount of the beads is required?
- What are the important points when designing a ligand?
- How are beads stored after the ligands are bound to them?
- Are there any methods other than HPLC for verifying whether or not ligand binding has been successful?
- How strong is the affinity for the proteins that are affinity purified?
- What is the purification efficiency?
- How is the cell extract prepared?
- Is there any problem with using frozen stock homogenate?
- How much protein supply is necessary?
- Can affinity purification be used with membrane proteins such as GPCRs and ion channels?
- There are many background bands. how can i reduce it?
- What should be done when a large number of bound protein bands are detected?
- Is it necessary to use the recommended buffer as the binding buffer?
- Why is it that both salt elution and boil elution are performed for elution?
- Does it happen that the band of bound protein becomes thin when the concentration of ligand is increased?
- Why can’t I see any bands of bound proteins?
- How long is the stable period of the ligand-immobilized beads?
- Is the optimal binding reaction time of 4 hours?
- I want to analyze bound proteins with MS, but what should I do if the target protein band is thin?
- How much protein can be analyzed by MS?

